Genetics BSCI 230, Spring 2003
University of Maryland
Riverbend DS Assocation Home Page » Resources » Term Papers & Reports » Genetics Term PaperCu/Zn Superoxide Dismutase: A Key Player in the Antioxidant Defense System |
|
Genevieve Houston-Ludlam Genetics BSCI 230, Spring 2003 University of Maryland |
Printed with the permission of the author |
| Cu2+ + O2-· → Cu+ + O2 | |
| Cu+ + O2-· + 2H+ → Cu2+ + H2O2 | |
| Net Reaction: | 2 O2-· + 2H+ → H2O2 + O2 |
2 H2O2 → 2H2O + O2The oxygen gas created in this reaction accounts for the bubbles seen when hydrogen peroxide is poured on a skin injury. Many bacteria do not have catalase, and the cells are destroyed, which is why hydrogen peroxide is effective against bacteria.
H2O2 + 2H+ → 2H2O
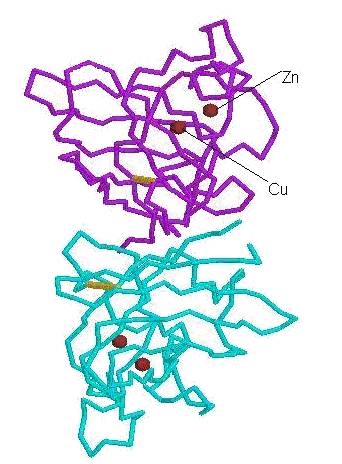 |
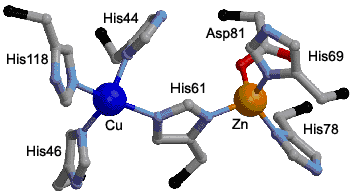 |